Argon helium or magnesium for example – Argon, helium, and magnesium, each possessing distinct chemical and physical properties, play crucial roles in various industrial, environmental, and medical applications. This exploration delves into the intricacies of these elements, uncovering their unique characteristics and highlighting their significance in shaping our world.
From the inert nature of argon to the exceptional thermal conductivity of helium and the reactivity of magnesium, these elements exhibit a diverse range of properties that determine their suitability for specific applications. Their abundance in the Earth’s atmosphere and crust, coupled with their potential environmental impact, further underscores the importance of understanding their behavior and responsible use.
Chemical Properties: Argon Helium Or Magnesium For Example

Argon, helium, and magnesium exhibit unique chemical properties that distinguish them from other elements.
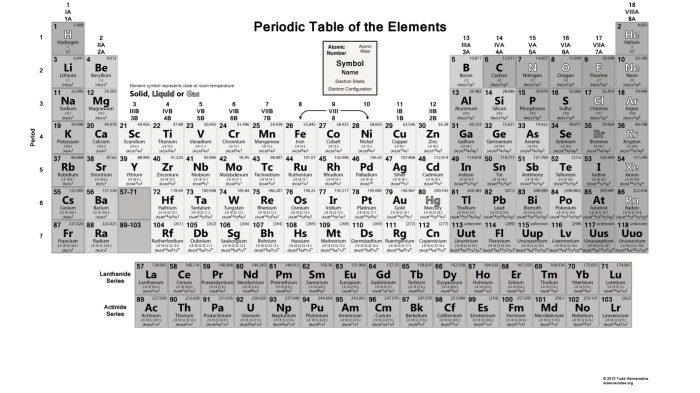
Argon is a noble gas with an atomic number of 18 and an atomic weight of 39.948. Its electron configuration is 1s 22s 22p 63s 23p 6, making it highly stable and unreactive. Argon does not form compounds under normal conditions.
Helium is also a noble gas with an atomic number of 2 and an atomic weight of 4.0026. Its electron configuration is 1s 2, making it the lightest and least reactive element known. Helium does not form compounds under normal conditions.
Magnesium is an alkaline earth metal with an atomic number of 12 and an atomic weight of 24.305. Its electron configuration is 1s 22s 22p 63s 2, making it highly reactive. Magnesium readily forms ionic compounds with nonmetals.
The following table summarizes the atomic number, atomic weight, and electron configuration of argon, helium, and magnesium:
| Element | Atomic Number | Atomic Weight | Electron Configuration |
|---|---|---|---|
| Argon | 18 | 39.948 | 1s22s22p63s23p6 |
| Helium | 2 | 4.0026 | 1s2 |
| Magnesium | 12 | 24.305 | 1s22s22p63s2 |
Physical Properties

Argon, helium, and magnesium exhibit distinct physical properties due to their unique atomic structures and bonding characteristics.
Argon is a colorless, odorless, and tasteless gas at room temperature. It is denser than air and has a melting point of -189.3 °C and a boiling point of -185.8 °C. Argon is a poor conductor of heat and electricity.
Helium is a colorless, odorless, and tasteless gas at room temperature. It is the lightest element known and has a melting point of -272.2 °C and a boiling point of -268.9 °C. Helium is an excellent conductor of heat and electricity.
Magnesium is a silvery-white metal that is lightweight and strong. It is a good conductor of heat and electricity and has a melting point of 650 °C and a boiling point of 1090 °C.
The following table summarizes the melting points, boiling points, and specific heats of argon, helium, and magnesium:
| Element | Melting Point (°C) | Boiling Point (°C) | Specific Heat (J/g·K) |
|---|---|---|---|
| Argon | -189.3 | -185.8 | 0.52 |
| Helium | -272.2 | -268.9 | 5.19 |
| Magnesium | 650 | 1090 | 1.02 |
Industrial Applications
Argon, helium, and magnesium have a wide range of industrial applications due to their unique properties.
Argon is used in welding, as it provides an inert atmosphere that prevents oxidation of the weld metal. It is also used in incandescent light bulbs and fluorescent tubes to prevent the filament from oxidizing.
Helium is used in balloons and airships due to its low density and non-flammability. It is also used in cryogenics, as it remains liquid at very low temperatures.
Magnesium is used in the production of lightweight alloys, such as those used in aircraft and automotive parts. It is also used in pyrotechnics and as a reducing agent in chemical reactions.
The following are specific examples of how argon, helium, and magnesium are employed in various industries:
- Argon is used in the production of stainless steel, as it prevents the formation of oxides on the surface of the metal.
- Helium is used in the cooling of superconducting magnets, as it has a very low thermal conductivity.
- Magnesium is used in the production of fireworks, as it burns with a bright white flame.
Common Queries
What is the most abundant noble gas in the Earth’s atmosphere?
Argon
Which element is known for its extremely low boiling point?
Helium
What is the primary use of magnesium in industrial applications?
Alloying agent for aluminum