Practice atom and the periodic table vocabulary – The practice of atom and the periodic table vocabulary is a fundamental aspect of chemistry, providing a systematic understanding of the elements and their properties. This guide delves into the intricacies of atoms, the organization of the periodic table, and the relationship between an element’s position and its chemical behavior.
Through a comprehensive exploration of the periodic table, we will uncover the patterns and trends that govern the properties of elements, enabling us to predict their reactivity and behavior in chemical reactions.
Definition of Atom
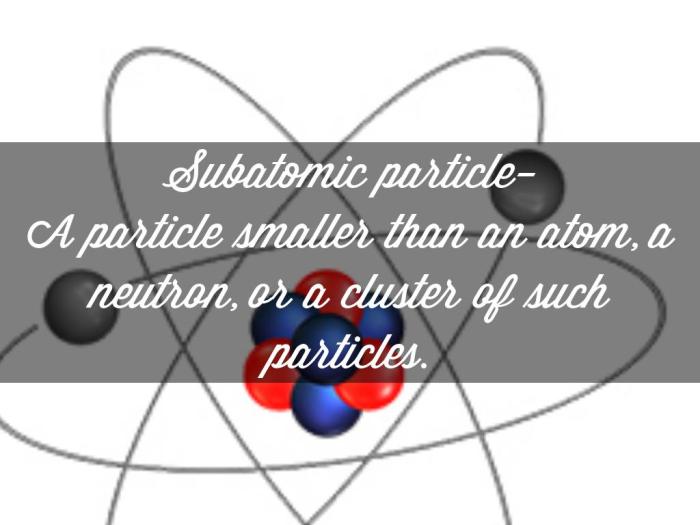
An atom is the fundamental building block of matter, consisting of a central nucleus surrounded by electrons. The nucleus contains protons and neutrons, while electrons orbit around it.
Atoms vary in size, structure, and properties. The smallest atom is hydrogen, with one proton and one electron. The largest known atom is oganesson, with 118 protons and 176 neutrons.
Atoms combine to form molecules and compounds, which are the building blocks of all matter. The properties of matter depend on the arrangement and interactions of the atoms that compose it.
Introduction to the Periodic Table
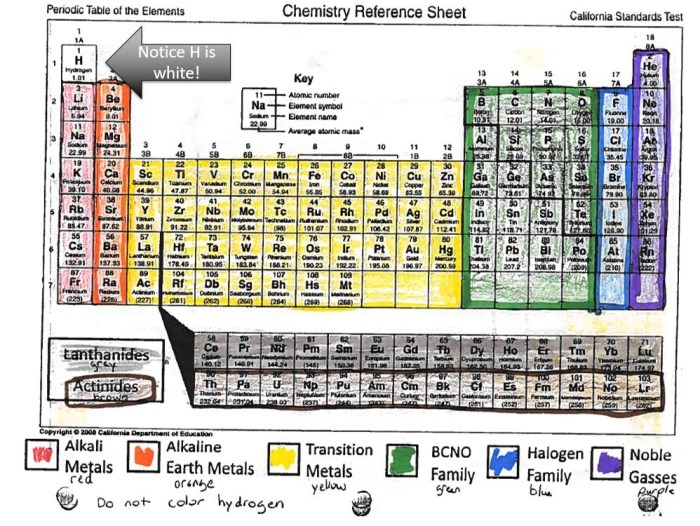
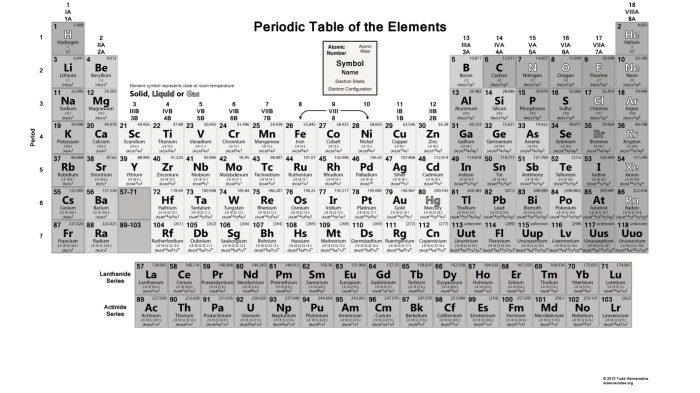
The periodic table is a tabular arrangement of chemical elements, organized based on their atomic number, electron configuration, and recurring chemical properties.
The periodic table consists of 18 vertical columns, called groups, and 7 horizontal rows, called periods. Elements in the same group share similar chemical properties, while elements in the same period have the same number of electron shells.
The periodic table is a valuable tool for predicting the properties of elements and understanding their behavior in chemical reactions.
Understanding Element Properties
An element’s position on the periodic table provides valuable information about its chemical properties.
- Atomic number: The number of protons in an atom’s nucleus determines its atomic number and identity as an element.
- Atomic mass: The sum of the masses of an atom’s protons and neutrons determines its atomic mass.
- Electron configuration: The arrangement of electrons in an atom’s electron shells determines its chemical reactivity.
These properties influence an element’s behavior in chemical reactions and its ability to form bonds with other elements.
Nomenclature and Symbolism
Elements are named according to the International Union of Pure and Applied Chemistry (IUPAC) guidelines.
- Element names are capitalized.
- Element symbols consist of one or two letters, representing the element’s name or its Latin origin.
- Element symbols are used to represent elements in chemical formulas and equations.
Chemical symbols are a concise and convenient way to represent elements and their properties.
Applications of the Periodic Table, Practice atom and the periodic table vocabulary
The periodic table has wide-ranging applications in various fields:
- Chemistry: The periodic table helps predict element properties, guide chemical reactions, and classify chemical compounds.
- Physics: The periodic table provides insights into atomic structure, electronic properties, and the behavior of matter.
- Materials science: The periodic table aids in designing and developing new materials with specific properties.
The periodic table is an essential tool for understanding the behavior of matter and chemical reactions.
FAQ Overview: Practice Atom And The Periodic Table Vocabulary
What is an atom?
An atom is the basic unit of matter, consisting of a nucleus surrounded by electrons.
How is the periodic table organized?
The periodic table is organized into groups (vertical columns) and periods (horizontal rows), with elements arranged based on their atomic number and chemical properties.
What is the relationship between an element’s position on the periodic table and its properties?
An element’s position on the periodic table can provide valuable information about its chemical properties, such as its reactivity, electronegativity, and ionization energy.