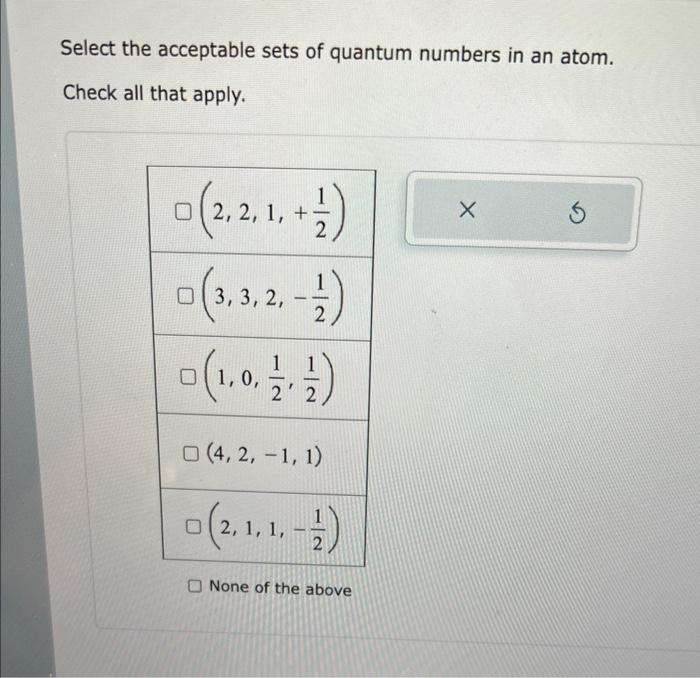
Select the acceptable sets of quantum numbers in an atom – Selecting Acceptable Sets of Quantum Numbers for Electrons in Atomsintroduces the fundamental principles governing the behavior of electrons within atomic structures. Quantum numbers, a cornerstone of atomic physics, play a pivotal role in determining the energy levels and chemical properties of elements. This comprehensive guide delves into the intricacies of quantum number selection, providing a foundation for understanding the complexities of atomic structure and its implications in chemistry and other scientific disciplines.
The concept of quantum numbers emerged from the groundbreaking work of physicists like Niels Bohr and Erwin Schrödinger. These numbers, denoted by n, l, ml, and ms, describe the unique characteristics of electrons within an atom, including their energy level, orbital shape, and spin orientation.
Understanding the rules that govern the selection of acceptable sets of quantum numbers is essential for comprehending the behavior of electrons and predicting the properties of elements.
Acceptable Sets of Quantum Numbers in an Atom: Select The Acceptable Sets Of Quantum Numbers In An Atom
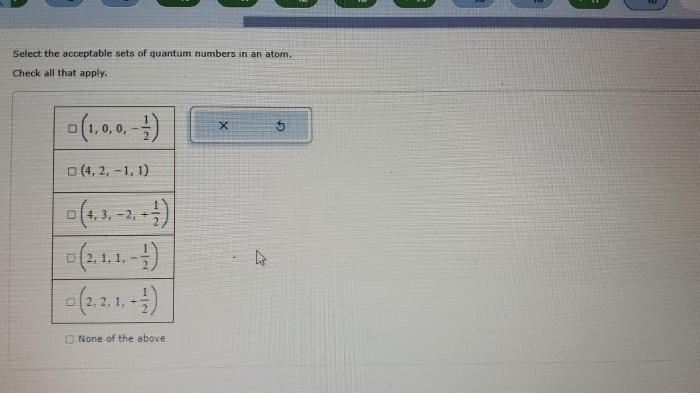
In atomic physics, quantum numbers are a set of four numbers that describe the state of an electron in an atom. They are essential for understanding the electronic structure of atoms and their chemical properties.
Quantum Numbers
The four quantum numbers are:
- Principal quantum number (n) : This number describes the energy level of the electron. It can take any positive integer value (1, 2, 3, …).
- Azimuthal quantum number (l) : This number describes the shape of the electron’s orbital. It can take any integer value from 0 to n-1.
- Magnetic quantum number (ml) : This number describes the orientation of the electron’s orbital in space. It can take any integer value from – lto l.
- Spin quantum number (ms) : This number describes the spin of the electron. It can take only two values, +1/2 or -1/2.
The following table summarizes the quantum numbers and their allowed values:
| Quantum Number | Symbol | Allowed Values |
|---|---|---|
| Principal | n | 1, 2, 3, … |
| Azimuthal | l | 0, 1, 2, …, n-1 |
| Magnetic | ml | –l,
|
| Spin | ms | +1/2,
|
The quantum numbers are related to the energy levels of an atom. The principal quantum number ( n) is the most important factor in determining the energy of an electron. The higher the value of n, the higher the energy level.
The azimuthal quantum number ( l) also affects the energy of an electron, but to a lesser extent than n. The magnetic quantum number ( ml) and spin quantum number ( ms) do not affect the energy of an electron.
Acceptable Sets of Quantum Numbers
Not all sets of quantum numbers are acceptable for an electron in an atom. The following rules must be followed:
- Pauli exclusion principle: No two electrons in an atom can have the same set of four quantum numbers.
- Aufbau principle: Electrons fill the lowest energy levels first.
- Hund’s rule: Electrons in the same orbital must have the same spin.
The following are examples of acceptable sets of quantum numbers:
- (1, 0, 0, +1/2)
- (2, 1, 0, -1/2)
- (3, 2, -2, +1/2)
The following are examples of unacceptable sets of quantum numbers:
- (1, 0, 0, -1/2)
- (2, 0, 1, +1/2)
- (3, 2, -2, -1/2)
(violates Pauli exclusion principle)
(violates Aufbau principle)
(violates Hund’s rule)
Methods for Determining Acceptable Sets of Quantum Numbers
The following steps can be used to determine the acceptable sets of quantum numbers for an electron in an atom:
- Determine the number of electrons in the atom.
- Use the periodic table to determine the electron configuration of the atom.
- Use the Aufbau principle to fill the electrons into the lowest energy levels first.
- Use Hund’s rule to determine the spin of the electrons in each orbital.
- Check to make sure that no two electrons have the same set of four quantum numbers (Pauli exclusion principle).
The following flowchart can also be used to determine the acceptable sets of quantum numbers for an electron in an atom:

Applications of Acceptable Sets of Quantum Numbers, Select the acceptable sets of quantum numbers in an atom
The acceptable sets of quantum numbers can be used to predict the chemical properties of an element. For example, the number of electrons in the outermost energy level of an atom determines its valence. The valence electrons are the electrons that participate in chemical reactions.
The acceptable sets of quantum numbers can also be used to explain the periodic table. The periodic table is a tabular arrangement of the elements, ordered by their atomic number. The atomic number of an element is the number of protons in its nucleus.
The acceptable sets of quantum numbers can be used to explain the trends in the periodic table, such as the increase in atomic number from left to right across a period and the decrease in atomic number from top to bottom down a group.
The acceptable sets of quantum numbers are also used in other fields of science, such as chemistry and physics. In chemistry, the acceptable sets of quantum numbers are used to explain the bonding between atoms. In physics, the acceptable sets of quantum numbers are used to explain the behavior of electrons in atoms and molecules.
User Queries
What is the significance of quantum numbers in chemistry?
Quantum numbers are crucial in chemistry as they determine the energy levels, shapes of orbitals, and spin orientations of electrons within atoms. This information is essential for understanding chemical bonding, predicting reactivity, and explaining the periodic trends observed in the properties of elements.
How does the Pauli Exclusion Principle affect the selection of quantum numbers?
The Pauli Exclusion Principle states that no two electrons in an atom can have the same set of quantum numbers. This principle governs the number of electrons that can occupy each energy level and orbital, influencing the electronic configuration and chemical properties of elements.
What methods are used to determine acceptable sets of quantum numbers for electrons?
Various methods can be employed to determine acceptable sets of quantum numbers, including using the periodic table, constructing orbital diagrams, and applying the Aufbau principle. These methods provide a systematic approach to assigning quantum numbers and predicting the electronic configurations of atoms.